Your location: Home>Technical Services> CDMO
CDMO
Yunbang Bio has a R&D center of 1,500 square meters in Changsha High-tech Zone Science Technology Park and a production base of more than 2,000 square meters in Huaihua, Hunan, equipped with professional facilities, equipment and operational skills. Our team is composed of industry experts with rich practical experience, familiar with drug molecular structure and process route design, can quickly carry out laboratory research & development and industrial scale-up production according to customer needs, provide drug product formulation and manufacturing expertise, a full set of comprehensive support services,a variety of standards and proprietary technologies.
1. Service Mode
Product delivery mode: including delivery of the final product, electronic data reporting. In addition to the agreement, relevant electronic data, including nuclear magnetic analysis, high performance liquid chromatography, mass spectrometry, gas chromatography, etc., are provided together.
2. Our Chemical Synthesis Capabilities
(1) Various small molecule compounds
(2) PEG Derivatives with various funcational group
(3) Medicinal chemistry and drug discovery
(4) Organometallic chemistry
(5) Heterocyclic chemistry
(6) Special reagents
(7) Scale-up production
3. Intellectual Property
1. Fully respect the customer's requirements, strictly follow the intellectual property protection agreement signed with the customer, and protect the customer's interests.
2. Improve the intellectual property management and protection system, including patent management system, trademark management system, technology and trade secret management system, etc., to enhance the core competitiveness of enterprises.
3. In any agreement signed with the customer, the company promises to keep the technical information provided by the customer confidential, and will not provide the information to a third party in any form without the customer's permission.
4. The company signs confidentiality agreements with relevant employees to ensure that all business secrets and customer information will not be disclosed to the outside world.
5. The company promises zero tolerance for any intellectual property infringement.
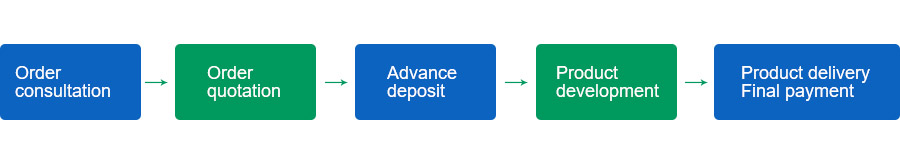
4. Service Process